Tetra Butyl Ammonium Chloride
Tetra Butyl Ammonium Chloride Specification
- Other Names
- TBAC; Tetrabutylammonium chloride
- Shape
- Crystalline solid
- Boiling point
- Decomposes before boiling
- Structural Formula
- [(C4H9)4N]Cl
- Taste
- Odorless
- Molecular Formula
- C16H36ClN
- Purity
- >=99%
- Solubility
- Soluble in water and alcohol
- Smell
- Odorless
- Density
- 0.89 Gram per cubic centimeter(g/cm3)
- HS Code
- 29239000
- Melting Point
- 37-40 C
- Form
- Powder
- Poisonous
- Yes
- Ph Level
- Neutral
- Molecular Weight
- 277.92 g/mol
- Storage
- Store in a tightly closed container, cool and dry place
- Classification
- Quaternary ammonium compound
- Chemical Name
- Tetra Butyl Ammonium Chloride
- CAS No
- 1112-67-0
- EINECS No
- 208-906-1
- Grade
- Reagent/Laboratory Grade
- Standard
- Analytical Grade
- Type
- Quaternary Ammonium Salt
- Usage
- Used as a phase transfer catalyst in organic reactions
- Main Material
- Tetra Butyl Ammonium Chloride
- Application
- Phase transfer catalyst, organic synthesis, laboratory chemicals
- Compatibility
- Compatible with most organic solvents
- Incompatibility
- Strong oxidizing agents
- Appearance
- White crystalline powder
- Stability
- Stable under recommended storage conditions
- Flash Point
- Not applicable (decomposes before boiling)
- Moisture Content
- <0.5%
- Shelf Life
- 2 years
- Hazard Class
- Not classified as hazardous under GHS
- Packing
- 25 kg drum / HDPE bag
Tetra Butyl Ammonium Chloride Trade Information
- Main Export Market(s)
- Central America, Asia, Eastern Europe, Western Europe, Middle East, South America, North America, Australia, Africa
- Main Domestic Market
- All India
About Tetra Butyl Ammonium Chloride
Tetra Butyl Ammonium Chloride with chemical formula of C16H36ClN is an organic chloride salt that comprises a tetrabutylammonium cation and chloride anion. It is a white crystalline powder that is soluble in water and polar organic solvents. It finds use in organic reactions that includes transferring anions from an aqueous phase to an organic phase, such as polymerization reactions. The other applications include its use as a corrosion inhibitor and as a biocide. It is a toxic compound and should be handled with care.
Versatile Phase Transfer Catalyst
TBAC is acclaimed for its efficacy in facilitating organic reactions where reagents must transfer between immiscible phases. Its compatibility with most organic solvents and excellent solubility make it an essential choice for chemical laboratories and industrial synthesis. The compound dramatically improves yields and reaction rates in processes requiring phase transfer catalysis.
Safe Handling and Storage
TBAC is non-poisonous and not classified as hazardous, making it safer to handle compared to many chemical reagents. For optimal stability, store TBAC in tightly closed containers kept in cool, dry environments. The product's two-year shelf life ensures lasting quality and reliability for scientific and industrial needs.
FAQ's of Tetra Butyl Ammonium Chloride:
Q: How should Tetra Butyl Ammonium Chloride be stored to maintain its stability and shelf life?
A: Store the product in a tightly closed container at a cool, dry place, away from strong oxidizing agents. Proper storage ensures the compound remains stable and effective for up to two years.Q: What is the primary usage of Tetra Butyl Ammonium Chloride in organic synthesis?
A: TBAC serves mainly as a phase transfer catalyst, helping facilitate reactions between compounds that typically reside in separate phases, thereby increasing reaction efficiency and yield.Q: When is TBAC most beneficial in chemical processes?
A: TBAC is especially useful during organic reactions that require enhanced interphase transfer of reactants, making it invaluable in processes such as nucleophilic substitutions and other laboratory syntheses.Q: Where is TBAC typically applied in industrial or laboratory settings?
A: TBAC is commonly utilized in chemical laboratories and manufacturing plants for organic synthesis, phase transfer catalysis, and as a laboratory reagent in research and development.Q: What precautions should be taken during the handling process of TBAC?
A: While TBAC is non-poisonous and not classified as hazardous, standard laboratory safety procedures should be followed, including avoiding contact with strong oxidizing agents and using personal protective equipment during handling.Q: Is Tetra Butyl Ammonium Chloride compatible with all types of solvents?
A: TBAC is compatible with most organic solvents and is highly soluble in water and alcohol, but it should not be mixed with strong oxidizing agents due to potential incompatibility.Q: What are the benefits of using TBAC in organic reactions compared to other catalysts?
A: TBAC improves yields by enabling efficient phase transfer, ensures greater reaction rates, and offers ease of handling and storage due to its stability, non-toxicity, and compatibility with various solvents.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Phase Transfer Catalysts Category
Tetra Methyl Ammonium Hydrogen Sulphate
Solubility : Soluble in water
Purity : 99%
Molecular Formula : C4H13NO4S
CAS No : 80584914
Grade : Other, Analytical Grade, Industrial Grade
Benzyl Trimethyl Ammonium Iodide
Solubility : Soluble in water and alcohol
Purity : >98%
Molecular Formula : C10H16IN
CAS No : 633976
Grade : Other, Laboratory/Reagent Grade
Trimethyl Sulphonium Iodide
Solubility : Soluble in water and polar solvents
Purity : >98%
Molecular Formula : C3H9IS
CAS No : 2181422
Grade : Other, Laboratory grade
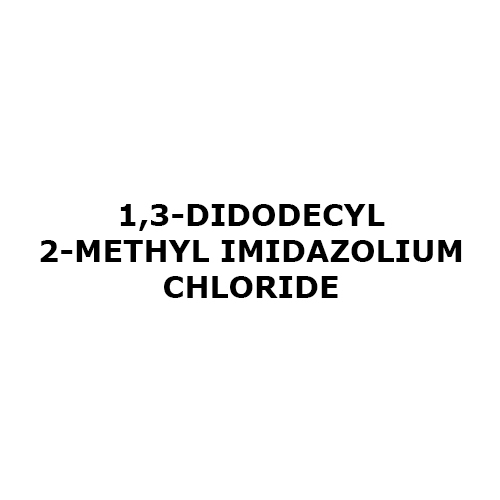
1 3-Didodecyl 2-Methyl Imidazolium Chloride
Minimum Order Quantity : 25 Kilograms
Solubility : Water
Purity : 98%
Molecular Formula : C28H58ClN2
CAS No : 70862656
Grade : Industrial Grade

 Send Inquiry
Send Inquiry





 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese